Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is often curable with frontline chemotherapy; however, pts with relapsed/refractory (R/R) disease and those who fail CAR-T therapy have poor overall outcome. Oral small molecule targeted agents can disrupt key survival pathways in DLBCL, but fail to induce deep or durable responses as monotherapy. We have previously shown that ViPOR is safe and active in DLBCL, with preferential activity in non-GCB DLBCL and HGBCL-DH (Melani et al. Blood. 2020). Synergistic activity has also been demonstrated with MMAE, the chemotherapy payload of Polatuzumab (Pola), with ViPOR in pre-clinical DLBCL cell lines (unpublished data). We hypothesized that additional genotoxic stress with Pola will further leverage efficacy and time-limited, cyclic dosing will limit the hematologic toxicity of the regimen.
Methods: R/R aggressive B-cell lymphoma pts with adequate organ function were eligible. In phase 1, a 3+3 design was used to determine the maximum tolerated dose (MTD) of 3 dose-levels (DLs) of Pola IV D2 and venetoclax (Ven) PO D2-14 (DL1: 1.4mg/kg + 600mg, DL2: 1.8mg/kg + 600mg, and DL3: 1.8mg/kg + 800mg) in combination with fixed-dose ibrutinib 560mg PO D1-14, prednisone 100mg PO D1-7, obinutuzumab 1000mg IV D1-2, and lenalidomide 15mg PO D1-14. An expansion cohort was included at the MTD for further analysis of safety and efficacy. ViPOR-P q21d x 6 cycles (C) was given without maintenance or planned consolidation. All pts received TLS, PCP, HSV, and G-CSF prophylaxis (ppx) with VTE ppx per investigator discretion. Baseline CT, PET, BM, and tumor biopsy were performed with CT scans after C2, 4, and 6 and PET after C6 or at time of suspected complete response (CR). Surveillance CT was performed q3m for 1y, q4m x 1y, q6m x 1y, then annually x 2y.
Results: 15 pts were enrolled and treated in the dose-escalation cohort; 3 at DL1, 6 at DL2, and 6 at DL3. Lymphoma subtypes included DLBCL NOS (60%) and HGBCL-DH (40%). In 9 DLBCL NOS pts, 11% were GCB and 89% non-GCB subtype by IHC. Three (20%) pts had transformed disease. Median age was 51y (range 39-83), with stage III/IV disease in 80%, elevated LDH in 80%, and >2 extranodal sites of disease in 67%. Nine (60%) pts had high-intermediate/high-risk IPI. Median prior therapies were 2 (range 1-6), with prior BTKi in 27%, Pola in 7%, and CAR-T in 20%. Ten (67%) pts were primary refractory (i.e., <CR to frontline tx) and 8 (53%) of pts were refractory (i.e., <PR) to last tx.
One dose-limiting toxicity (DLT) of prolonged G4 thrombocytopenia occurred at DL3, but no other DLTs were noted. Pola 1.8mg/kg and Ven 800mg were identified as the MTD and safe doses for use in the expansion cohort. Heme AEs were most common and included G3-4 (% cycles) thrombocytopenia (17%), anemia (7%), and neutropenia (5%). No cases of febrile neutropenia occurred across 58 cycles. The only non-heme G3-4 AE occurring in >10% pts was hypokalemia (29%), with all pts experiencing any grade hypokalemia requiring replacement. Other common non-heme AEs (% pts) included diarrhea (71%), increased LFTs (64%), nausea (50%), hypomagnesemia (43%), and rash (43%). VTE occurred in 7% with no a.fib or major bleeding observed. No events of TLS or treatment-related mortality occurred. Dose reductions and delays occurred in 3% and 5% of cycles, respectively. Of 12 pts off-tx, 7 completed all 6C, 3 experienced disease progression, and 1 each came off-tx per investigator discretion or unacceptable AE.
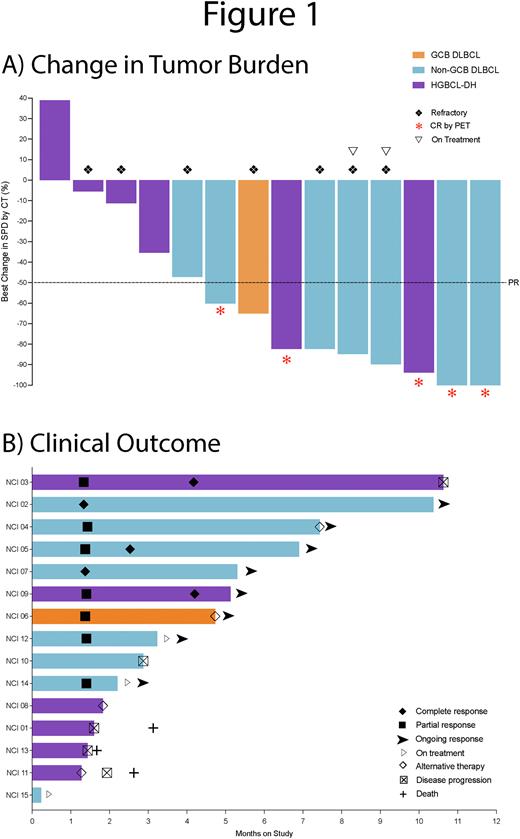
Of 15 enrolled pts, 14 are evaluable for response with an overall response rate (ORR) and CR rate of 64% (9/14) and 36% (5/14), respectively (Fig 1A). With a median potential f/u of 7.4m, median TTR in responding pts was 1.35m, with 8 (89%) of 9 total responses ongoing (Fig 1B). One HGBCL-DH pt relapsed by imaging at 10.6m, 2 PR pts received consolidative XRT with allo-HSCT, and 2 PR pts remain on-tx. PFS and OS at 6m was 69.8% and 75%, respectively.
Conclusion: ViPOR-P is safe without significant additional toxicity compared to ViPOR, and Pola 1.8mg/kg and Ven 800mg were taken forward in the ongoing expansion cohort. Most common G3-4 AEs were hematologic with no febrile neutropenia observed with the use of G-CSF ppx. Fixed-duration ViPOR-P x 6C without maintenance led to CR in 5 (42%) of 12 off-tx DLBCL pts with 80% CRs ongoing from 3.7-9.3m. A randomized phase 2 trial is planned to further elucidate the safety and efficacy of Pola in combination with ViPOR across R/R DLBCL and within pre-defined molecular and genetic subtypes.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
ViPOR-P is not approved for use in aggressive B-cell lymphomas.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal